Everything which is seen around us is made up of one or the other thing. They have different shapes, structures, and properties. Some are hard, some are soft and some are in the form of a gel. Do you know why they are different from each other and what they are called?
It is a matter
Let’s learn more about the matter
What is a Matter?
The matter is something that makes up all the things around us. Everything which we are seeing around us is matter. so we can say the matter is something that occupies space and has some weight is called matter.
Or
Anything that has mass and some volume is called matter.
By Mass we mean the amount of matter present in any substance or we can say the total weight of a substance. sometimes it happens the things which seem small to us are having more mass as compared to the others which seem big to us. For example, A big balloon filled with helium gas is having less mass on the other hand a small ball made of lead is having more mass.
By Volume we mean the amount of space takes up by any substance. Like small, big, few little all these tell us the volume of a substance. It is also called capacity. Every matter filled up space in their own way.
All Matters are made up of atom, These atoms are consists of a small subatomic particle like electron, neutron, and proton. A group of atoms forms a molecule which is the building block of all kinds of matter. Molecules together form a compound. For example, One molecule of Sodium and Chlorine forms Sodium Chloride.
Na + Cl = NaCl
Broadly Matter exists in five forms Solid, Liquid, Gases, Plasma, And the fifth state Bose-Einstein Condensate
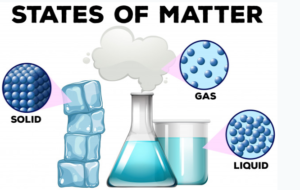
States of Matter
1.Solids
Characteristics of Solid
- Molecules are tightly packed with each other.
- Solids are hard, rigid, and have definite shape and volume.
- Solids have high density
- The electrons of each atom are constantly in motion, they have small vibrations but still, they are fixed in their position.
- Example-Table, chair, and wood, etc.
2.Liquids
Characteristics of Liquid
- Molecules are less tightly packed as compared to solids.
- Molecules can move around freely and hence liquid can flow
- Liquid has a definite volume but no a definite shape as they take the shape of the container in which they are poured
- For Example-Milk, Juice, Water, etc.
3.Gases
Characteristics of Gas
- Molecules are far apart from one another and can move freely away from one another.
- The molecules in a gas move much faster than molecules in a solid/liquid.
- Gases can therefore flow easily and have no definite shape or volume.
- For Example-Oxygen, Nitrogen and Carbon dioxide etc.
Molecular structure of Solid, Liquid and Gases

4.Plasma
Characteristics of Plasma
- Plasma consists of highly charged particles with extremely high kinetic energy.
- These are common state of matter in the universe., Stars are superheated balls of plasma.
- They are used to make glowing signs by using electricity to ionize.
- Example-Nobel gases like Neon, Helium, Krypton, Argon, Xenon are ionized by using electricity to convert them into a plasma state.
5.Bose -Einstein Condensate-
Characteristics of BEC
- It is the man-made state of matter created by scientists in 1995 by using a combination of lasers and magnets.
- The fifth state of matter in which separate atom or subatomic particles cooled down to absolute zero.
- Property of superfluid that is which can flow without friction.
Out of these five states of matter, the first three states of matter are the commonly seen states of matter which we can observe around us.
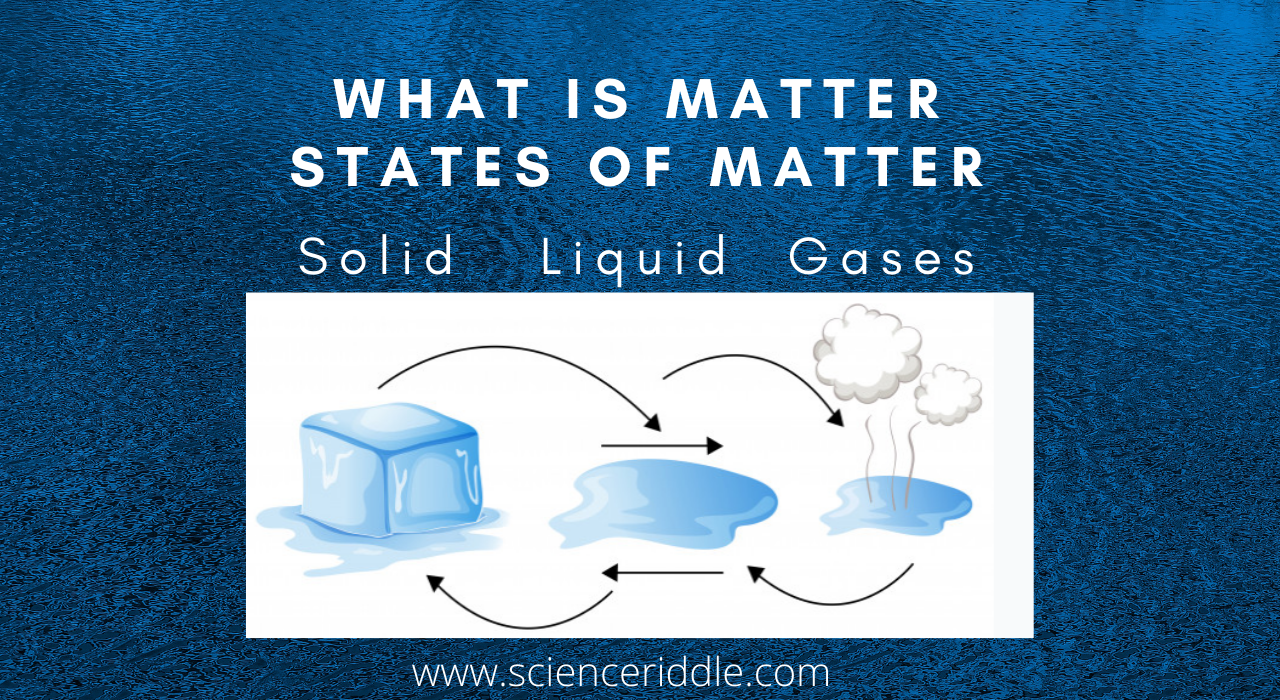
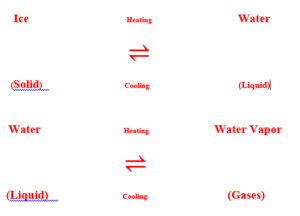
Three states of matter are also interchangeable states of matter by the physical change in the state of matter they are interchangeable like for example, water can exist in all three states of matter.
Interconversion of states of matter
Water can exist in three states of matter Solid, Liquid, and Gases.
Try to observe all these different states of matter in your surrounding.
Read about Microbes | Types of Microbes